A Safe Way to Treat Unwitnessed Strokes
Orange County, CA - April 26th, 2018 -
Researchers at the Massachusetts General Hospital (MGH) may be able to expand the number of stroke patients that can safely be treated with tissue plasminogen activator (tPA). tPA was approved by the Food and Drug Administration in 1996. It is a potent blood thinner that’s able to restore blood flow to the brain and limit any possible damage from a stroke.
Unfortunately, only 4 percent of stroke patients receive the medication. In order for it to work, the patient must receive the medication within the first few hours of symptoms. The window for this treatment can be up to 24 hours for some patients, however, the earlier they receive it, the better. TPA is not approved to use on patients whose symptoms aren’t visible and it prevents them from receiving the medication.
According to the press release, “U.S. Study eligibility was determined by a mismatch between two types of MR imaging studies – FLAIR, which typically only shows stroke effects on brain tissue after several hours of reduced blood flow, and diffusion-weighted imaging (DWI), a technique first applied to stroke patients at the MGH-based Athinoula A. Martinos Center for Biomedical Imaging that is extremely sensitive to changes beginning in the first minutes or hours after stroke onset.”
Abnormal brain tissue that shows up on the DWI, but not FLAIR has shown patients that were four hours or less after their first experience of any symptoms. Co-lead author Ona Wu, PhD of the Martinos Center states “That discrepancy provides a snapshot of tissue evolution as the stroke progresses in the first few hours, and that is the pattern we used to select patients for treatment, since they were likely to be similar to patients with known symptom onset who have benefited from tPA. Essentially we used an MR Witness – the name of the trial – to identify patients who might be treated with tPA because their strokes had not progressed to the point of irreversible injury.”
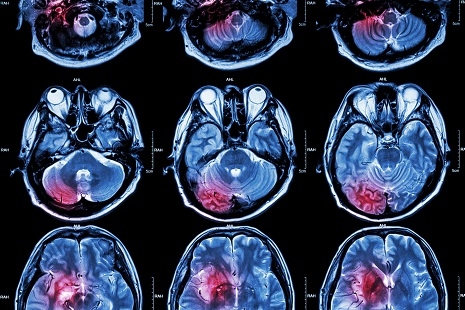
The outcomes for study participants are similar to the patients who previously received tPA within the 4 hours of witnessed strokes. Patients went to sleep normal and woke up with stroke symptoms which are known as “wake-up strokes”. The research team calls this qDFM for “quantitative DWI FLAIR Mismatch.” With this development, researchers hope to conduct a placebo-controlled trial that will also compare MR with CT imaging for the identification of patients with unwitnessed strokes. They then could safely and effectively be treated with tPA as specified in the press release.
Doctor Lee Schwamm the vice chairman of the Department of Neurology at MGH says “If that phase 3 study is successful, it would lead to a paradigm shift in the way acute stroke patients are treated. Rather than treating based on the number of hours since a stroke began, we can treat based on how much damage the stroke has already caused and how much brain can still be saved. Because the imaging methods we will use in our phase 3 study are available on an MRI or CT scanner in use today, if the results of that trial are positive, the approach could be put into practice immediately.”
Contact Ampronix:

Email: info@ampronix.com
International Sales: +1 949-273-8000
Domestic Sales: 1800-400-7972 for US and Canada
Follow Us:
Share This Article:
View our Product Catalog Online Here
About Ampronix
Ampronix is a renowned authorized master distributor of the medical industry's top brands as well as a world-class manufacturer of innovative technology. Since 1982, Ampronix has been dedicated to meeting the growing needs of the medical community with its extensive product knowledge, outstanding service, and state-of-the-art repair facility. Ampronix prides itself on its ability to offer tailored, one-stop solutions at a faster and more cost-effective rate than other manufacturers. Ampronix is an ISO & ANSI/ESD certified facility. To learn more go here.
A Safe Way to Treat Unwitnessed Strokes Orange County, CA – April 26th, 2018 – Researchers at the Massachusetts General Hospital (MGH) may be able to expand the number of stroke patients that can safely be treated with tissue plasminogen activator (tPA). tPA was approved by the Food and Drug Administration in 1996. It is a […]



